Project case study: Operating model digitization using the example of a pharma company

The business process (SOP) landscape of a leading pharma corporation posted to be a major pain point despite several prior process improvement initiatives. A complete operating model transformation was now necessary since management of business processes and GxP training in a paper-based and manual fashion was significantly limiting the ability to digitally transform the company while – at the same time – staying compliant.
Please note, that all information presented in this case study is purely fictitious and has been significantly changed for demonstration purposes only, so that no conclusions can be drawn about our client and no confidential content is conveyed.
Industry background
The pharmaceutical industry is highly regulated. Companies have to ensure that they reliably comply with all regulations, such as from the European Medicines Agency (EMA) or the US Food and Drug Administration (FDA). This requires continuous training and education of employees, often in the form or using so called SOPs (Standard Operating Procedures). It is currently common industry practice that the majority of training measures are simply extended to all employees in order to be on the safe side as violations of regulations can have serious financial consequences for any pharma corporation. As a result employees have to read numerous SOPs at the start of a project or when entering a company or be trained on it. Due to the sheer volume, however, a lot of content is quickly forgotten, which often requires a re-training. To put it into a nutshell: Today, a very inefficient approach that also ties up a lot of expensive human resources, as the majority of employees especially in drug development of any pharma company have at least a masters degree or even a PhD.

Corporate challenges
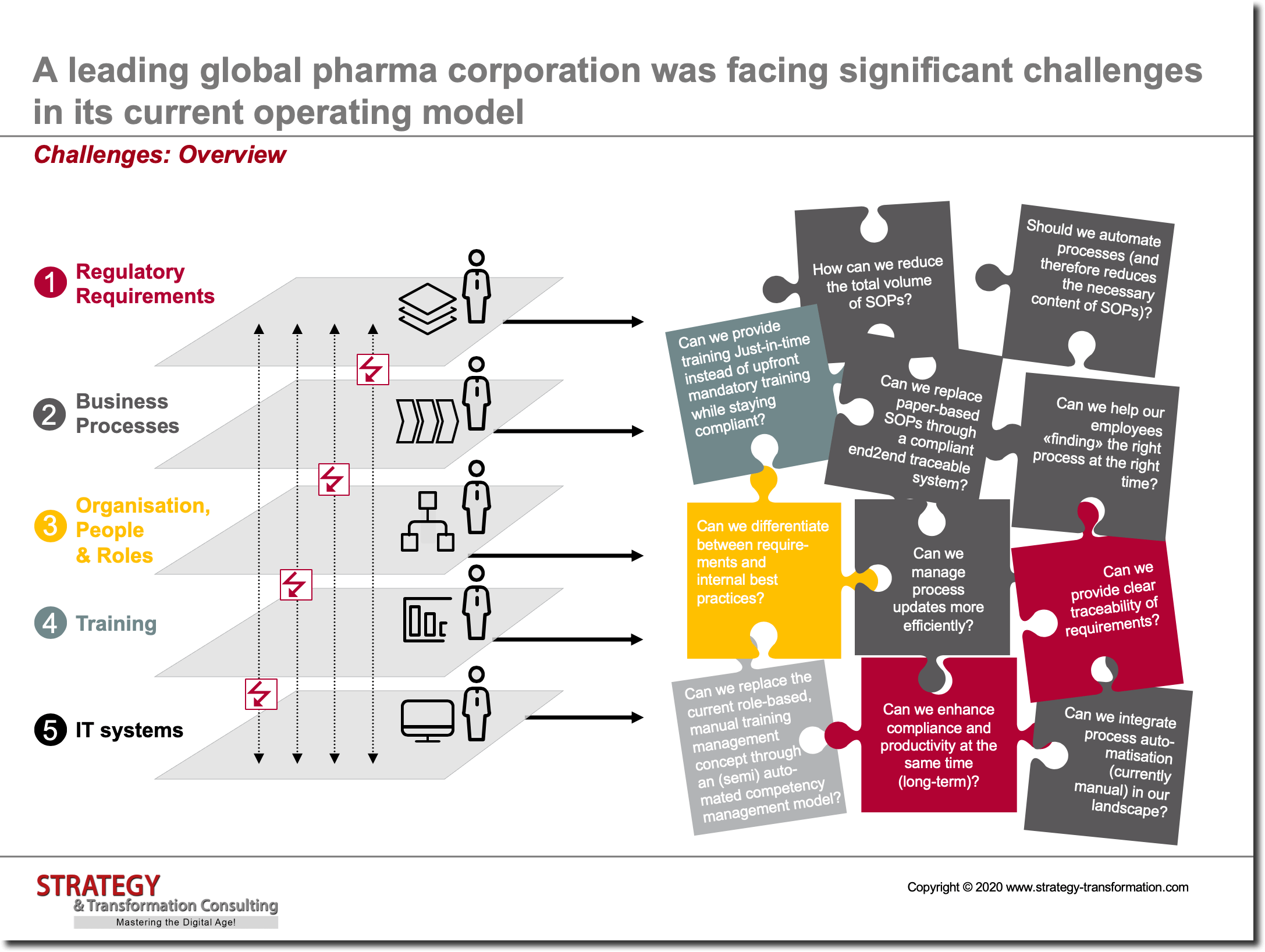
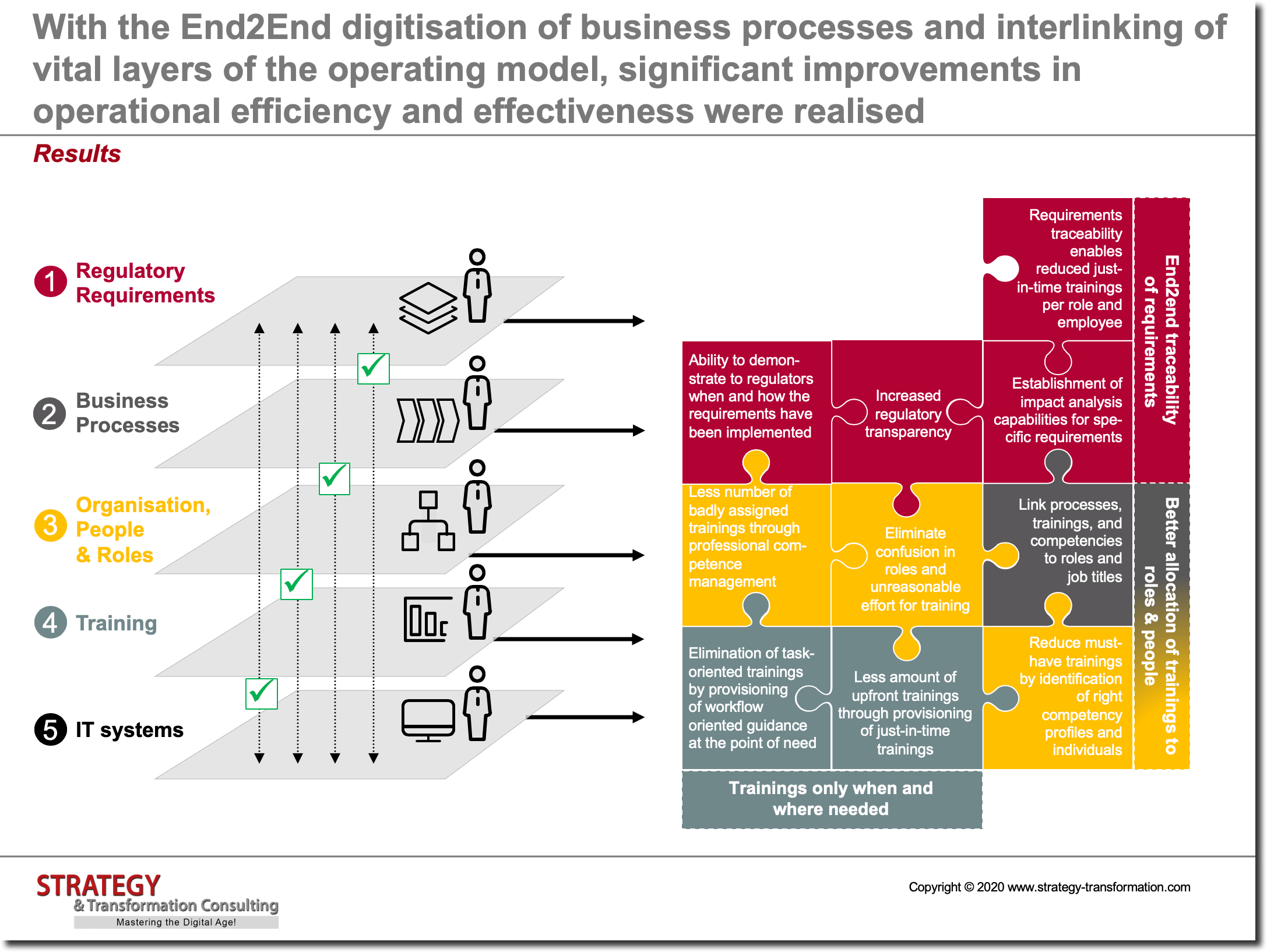
A leading global pharma corporation was facing significant challenges in its current operating model. One significant challenge was a very high amount of upfront trainings and despite the high investment, ever increasing audit findings. As part of a detailed analysis which we conducted in a first project phase, we found out that the current impediments and issues are due to an insufficient integration and interlinking of five levels of the operating model:
Regulatory Requirements
- Missing requirements traceability of regulatory directives caused tremendous training effort per employee
- Lacking end2end traceability and interlinkages
- Most documents were paper based and difficult to manage
Business Processes
- No uniform end2end business process logic
- Insufficient user specific views on business processes
- Little use of IT for process automatisation or guidance
- Unsatisfactory business process governance
Organization, People & Roles
- Many different role definitions in place and tremendous effort for low training value
- No end-to-end interlinkages between organizational, business process and training roles
- No consistent competency management in place
- Strong thinking in silos
Trainings
- high upfront training volumes especially for new joiners
- big time delay between training and usage of learnings
- long training times due to detailed and difficult to comprehend content
- high number of wrongly assigned trainings
- little proof on knowledge transfer to employees
IT Systems
- Highly complex IT landscape with missing end2end logic
- No big technology innovations visible
- Outdated software development processes
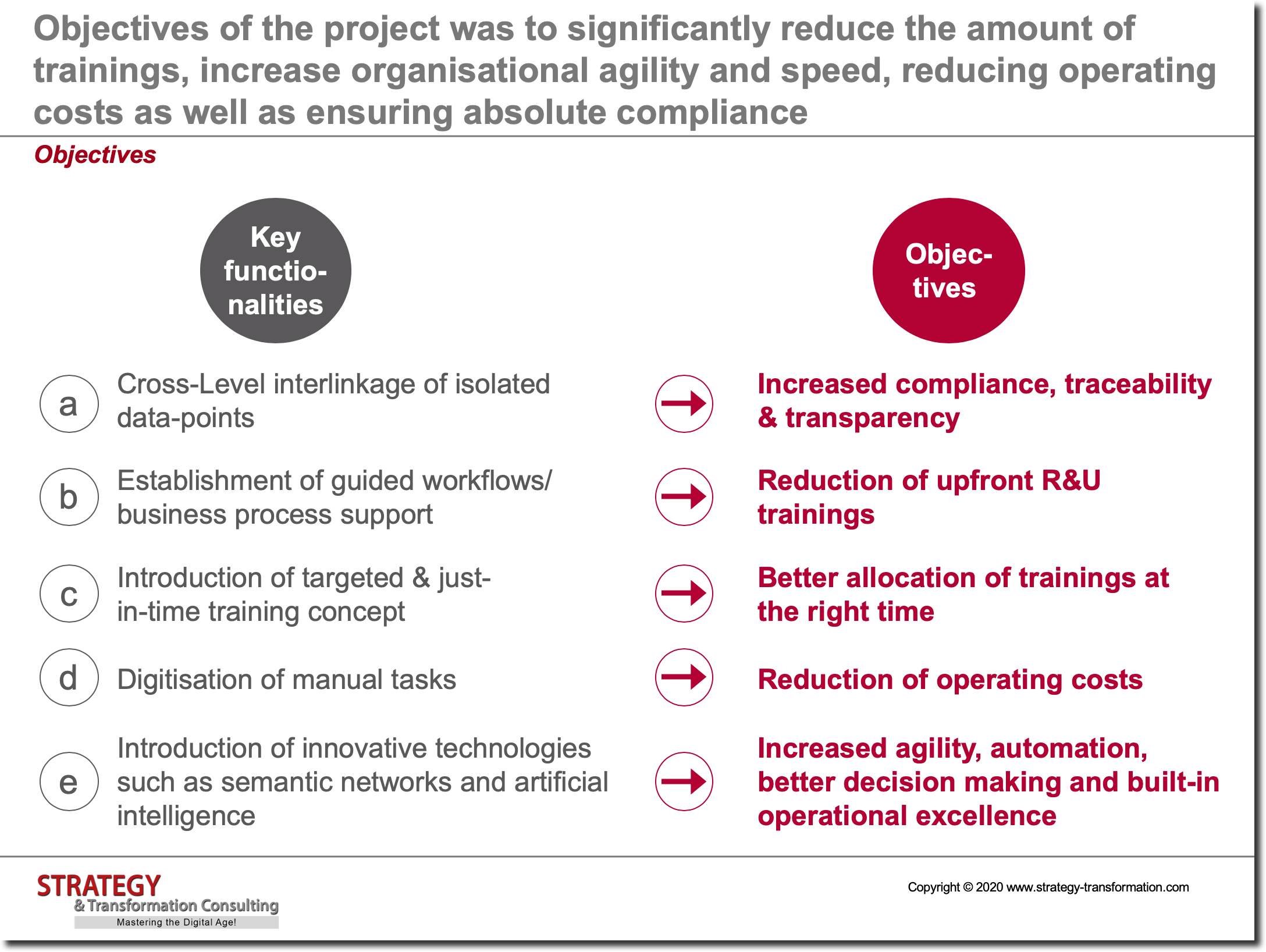
Project objectives
Objectives of the project was to significantly reduce the amount of trainings, increase organisational agility and speed, reducing operating costs as well as ensuring absolute compliance.
Conceptual approach
In order to be able to assign the right information and training to employees – and to generally relief employees from non-value generating tasks – it is necessary to holistically present and connect various levels of the operating model to each other: Regulatory requirements, business processes, organization / people / roles, trainings and IT systems. In addition, regulatory requirements which influence business processes must be viewed individually and divided into two fractions: Purely regulatory and business-related instructions. Quality management must ensure that regulatory directives are correctly understood and relevant SOPs – which are derived from directives – are thoroughly checked. The associated business process landscape must also be optimized, including levers for process optimization as well as automatization reaped. Since a large number of processes are involved here, this is a long-term task which must be supported by all departments. Among other things, this also requires an appropriate commitment from top management so that all departments release enough people who are familiar with the individual processes.
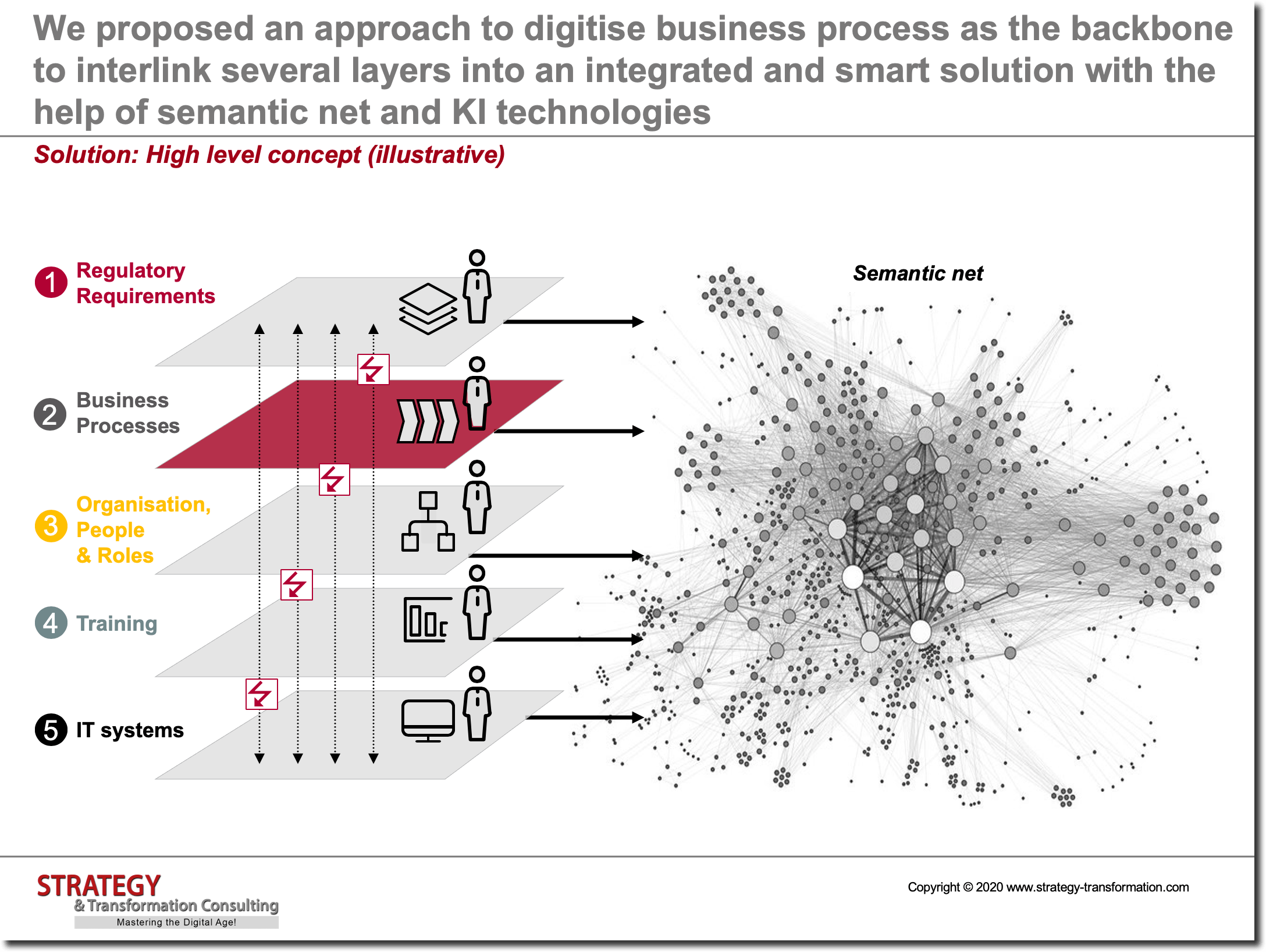
Moreover, organizational structures, people and their roles in the company also play an important role in this area, since the main users and drivers of business processes – apart from IT systems – are located in this area. The HR department can support here above all by providing a clear picture of the as-is skills and qualifications present in the company as well as provide an overview on future additional requirements and the way in which trainings are carried out in the firm. The specific training program for fulfilling regulatory requirements and for generally improving employee qualifications must be designed in coordination with the associated business process, employees, IT infrastructure, applications, hardware, IT services, data models, etc. It must also be geared towards supporting employees in fulfilling their business processes to develop individual skills/capabilities to successfully meet current and future demand. It is particularly crucial to create a good interconnection between all levels of the operating model – both in terms of better efficiency as well as adequate transparency and good traceability. The latter, in particular, is a prerequisite for being able to prove – at any given time – that all employees have completed all required trainings for a specific task and their actions are in compliance with associated regulations. On the other hand, professional traceability is also crucial in order to recognize which steps in a process are necessary from a regulatory perspective and which steps can be established from best practice scenarios within the company, to secure competitive advantages. In addition, organizational rules for controlling of clinical studies and projects must be standardized across the entire company. This is particularly challenging for those companies that have emerged from a merger and first complete their post merger integration process prior to additional transformation approaches.
Technical solution
As a solution to the challenges described in this article – together with our information technology consulting partner Piterion – we did not only develop a digital operating model for the pharma industry on a conceptual basis, but also an IT implementation.
Our operating model is digitally fully integrated, which means that the individual processes and other levels of the operating model – from regulatory and internal requirements to training and business processes to the roles and skills of individual employees – are transparently combined in the application. Various connection options enable data to be obtained from a wide variety of existing IT systems, such as individual learning management systems, from business process management engines to enterprise resource management solutions or customer relation management applications. As a further data source, individual systems within the company are also possible to integrate at any time.
Users receive an overview of their current projects and tasks after logging into their dashboard on their PC or mobile devise (tablet and smartphone), which is connected to the company IT architecture via single sign-on. The tasks assigned to the logged-in person are displayed in a personalized manner. The application also shows all trainings required in the near future. In addition, there are further trainings, which are not compulsory, but can be chosen by the employee as nice to have option.
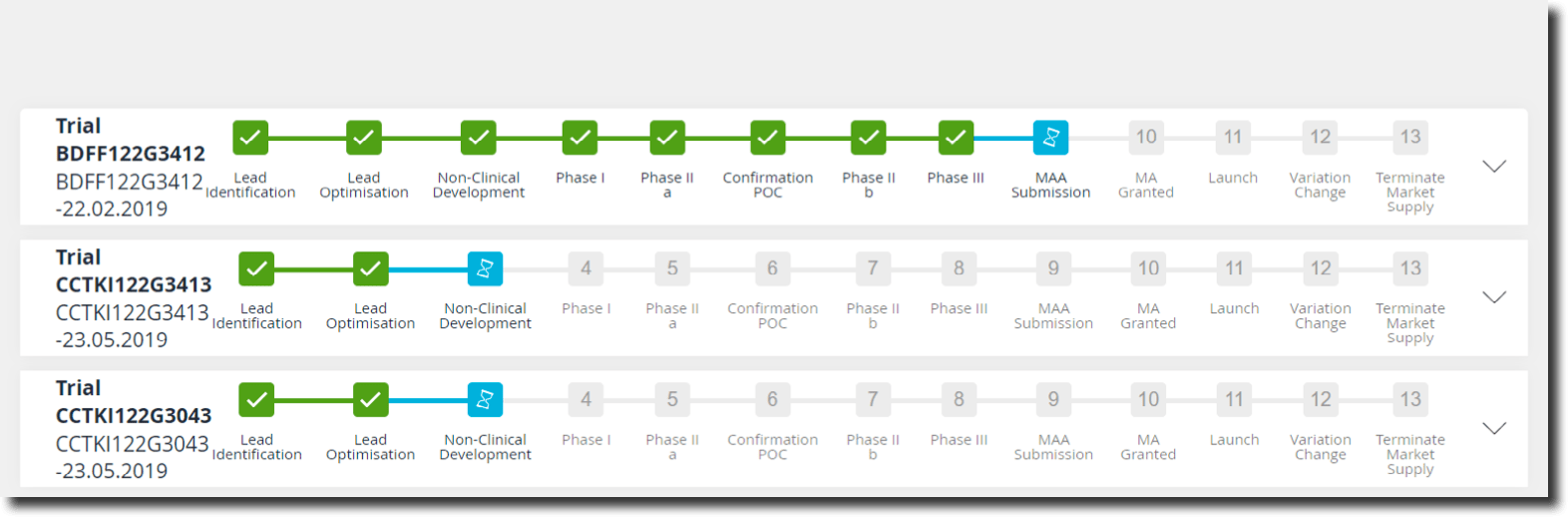
Under the „My Trials“ tab you will find a color-coded overview of the various phases of the current clinical studies that are of interest to users. A detailed overview available for each clinical study shows further information such as general data and study ID as well as outstanding tasks, precisely tailored to the respective organizational role of the user. Depending on the clinical study, other documents or actions may also be stored here. Users can link these to the respective originator systems (e.g. their email client). They enable them to take appropriate actions. This can range from simple connections such as creating an Outlook appointment with relevant participants to complex laboratory management systems, provided the IT systems to be connected have a modern interface. Due to the connection of regulatory requirements with respective business process steps, a validation can be carried out at each individual step. Country-specific regulatory requirements can also be dealt with. For example, a specific process step in some countries may enter the next phase earlier due to specific regional requirements. If necessary, confirmations which need to be tracked during such a process step can be recorded, these can be directly integrated into the process by means of a just-in-time read confirmation.
One real advantage of the technical solution is, that it recognizes the skills and knowledge as well as all completed trainings of users through the connections across the various company IT systems. If a process step or task is started, just-in-time training can easily be implemented. In practice, this often means only a short interruption of daily work routines of the respective user in the form of quick read & understand training. Cumbersome training sessions planned well in advance are no longer necessary!
When assigning tasks, the study lead also has the option of obtaining suggestions for suitable colleagues suggested by the IT system. The application only suggests people who are available and cover the required knowledge defined for the respective role. In addition to the master data, the user profile also contains relevant role descriptions with responsibilities, key performance figures, personal development pathways and dedicated training measures pending. The application is smart enough to also suggest a possible development path for the employee from the current to the next higher position (or any other position) and necessary training courses to be completed in order to acquire needed skills for the desired higher position. Users always receive suggestions for specific training courses including possible dates when the training is held. At this point, superiors also have the opportunity to highlight certain job profiles, they strategically would like to promote more strongly.
Prototype / showcase
As the developed concept – which we call „Digitally Integrated Pharma Operating Model (DIPOM)“ – was novel for the pharma industry, a pilot phase (after the conceptual project phase had been completed) was proposed to develop a showcase applying various tools and technologies by digitalizing end-to-end process to better understand specific challenges before investments are made for scale-up. The concept was using other industries as role model, which are more advanced in the digitalization of their processes. Leading automotives OEMs, aerospace companies and global financial services institutions were visited during a first scoping phase of the project. The prototype was built on the basis of a real operational problem. In the following we briefly describe the optimized and digitized workflow of Deborah, a study lead at a leading pharmaceuticals company. Please note, that the showcase is purely fictitious and has been significantly changed for demonstration purposes, so that no conclusions can be drawn about our client and no confidential content is conveyed.
Deborah has been recently promoted as study lead for the trial XYZ100AB and one day in the morning on her way to the Campus, she receives on her phone a push notification that tells her to compile all relevant documents needed for the submission to EMA and FDA (the relevant regulatory authorities in the EU and US). Since this is the first time that Julia is performing this task as a study lead, this information is mandatory for her and she needs to do it in the next few days.
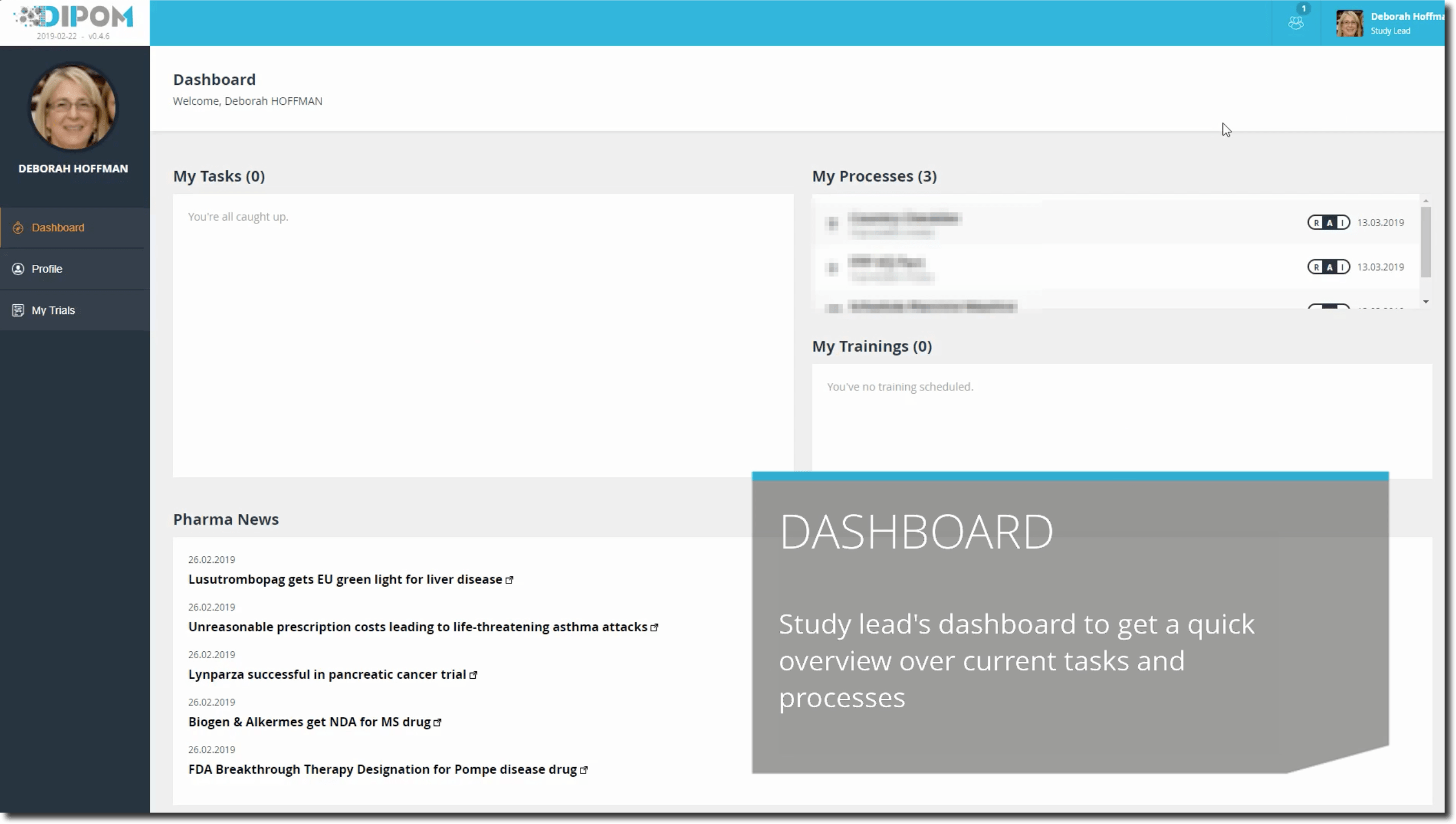
Deborah, as soon she arrives in the office, logs into her computer and opens her dashboard, performs the training assigned to her and swipes to the right to indicate that she read and understood the content. After that, she checks the overall status of the XYZ100AB trial for which she is accountable for and reviews the upcoming tasks assigned to her.
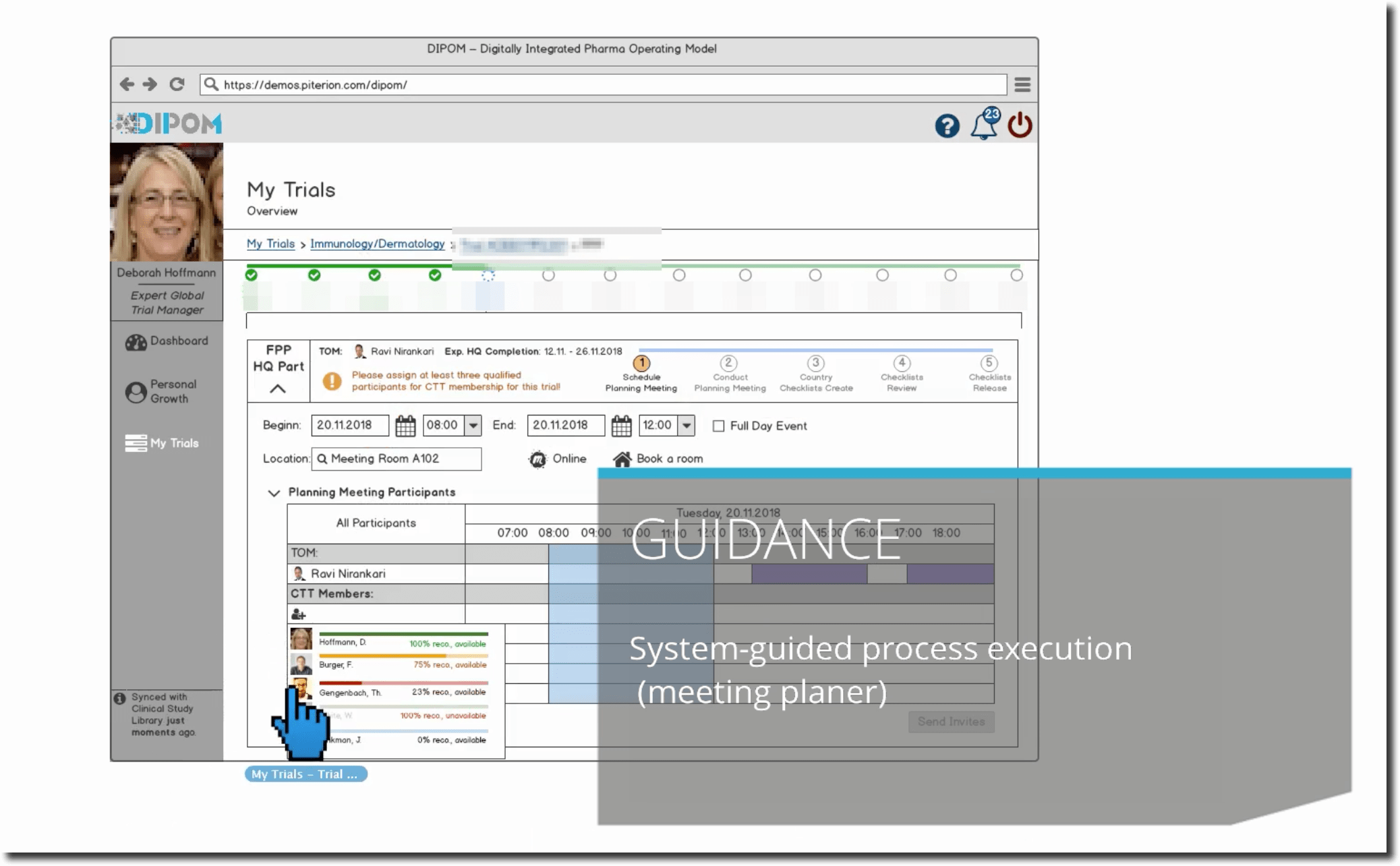
Immediately the IT system indicates that a bottleneck is coming up in the next two weeks on the trial that she is leading: Julian, one of her direct reports assigned as Clinical Document Specialist to the XYZ100AB trial changed job and another employee with the same skills as Clinical Document Specialist is not available at the moment.
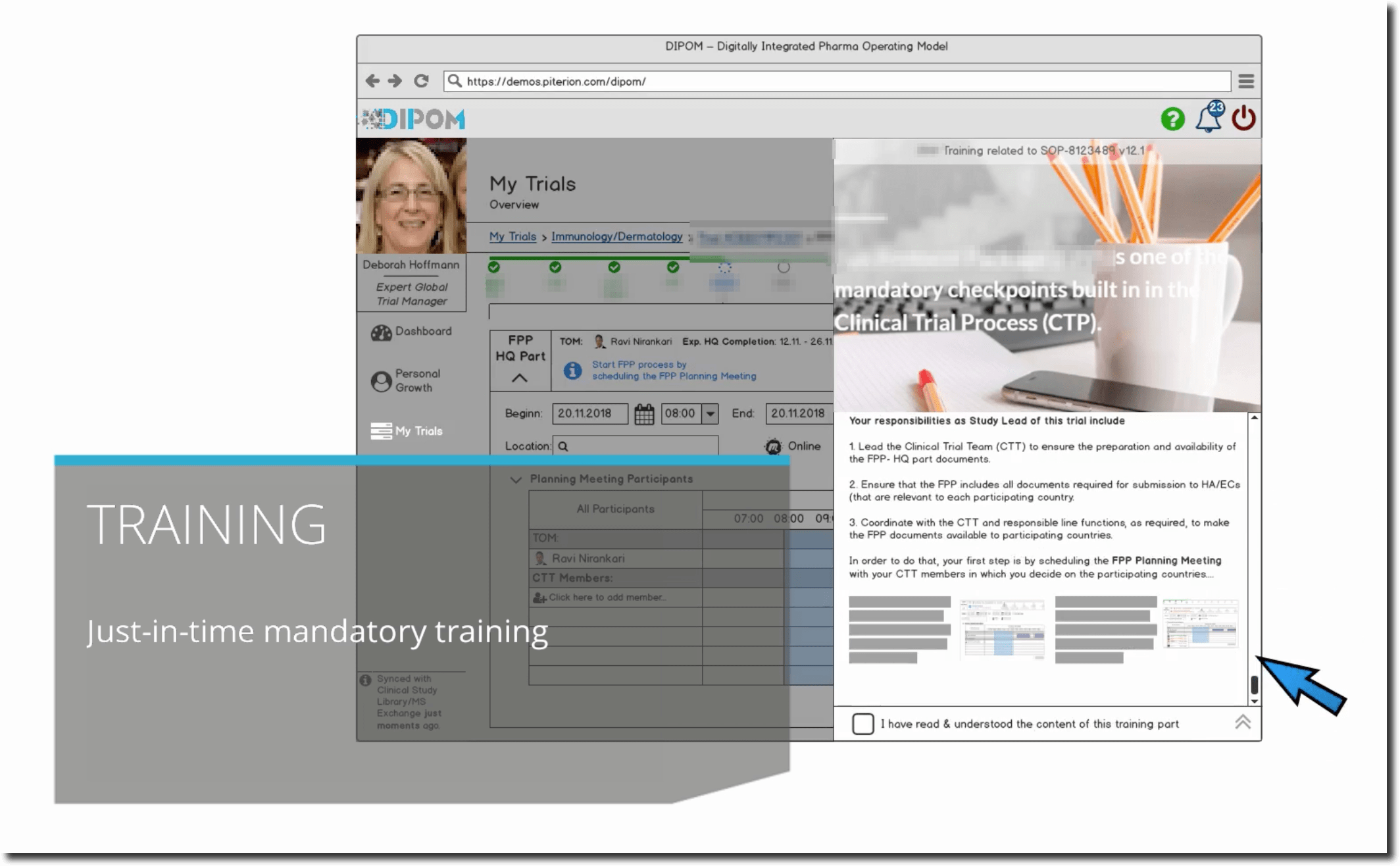
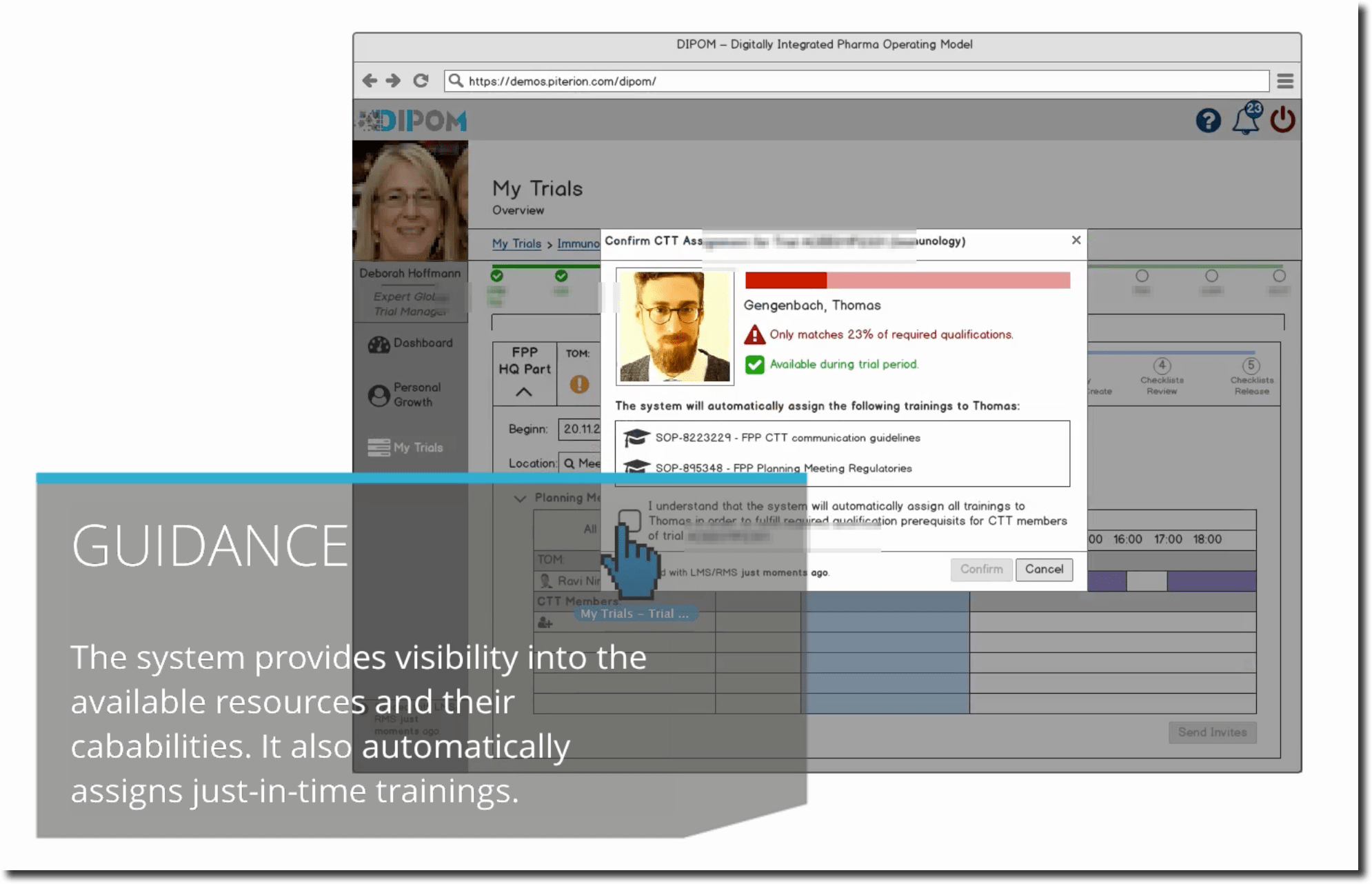
However, the dashboard indicates to Deborah that Thomas, who is also a Clinical Document Specialist, would be available to take over, but he misses one mandatory training (Trial Submissions Overview / Introduction Training). Hence, after much consideration, she assigns Thomas to the respective training through her dashboard and the relevant learning management system sends automatically the notification to him. Thomas immediately sees the notification in his own dashboard and takes on the training. Afterwards the systems does not show the notification anymore.
The next day, Deborah receives from the system another notification to perform a new regulatory training regarding patient protection. She is wondering why she is receiving this notification as she took the training 6 months ago. However, immediately she realizes that the regulatory requirements have been changed recently and all employees received the notification through the system to be trained on this specific topic.
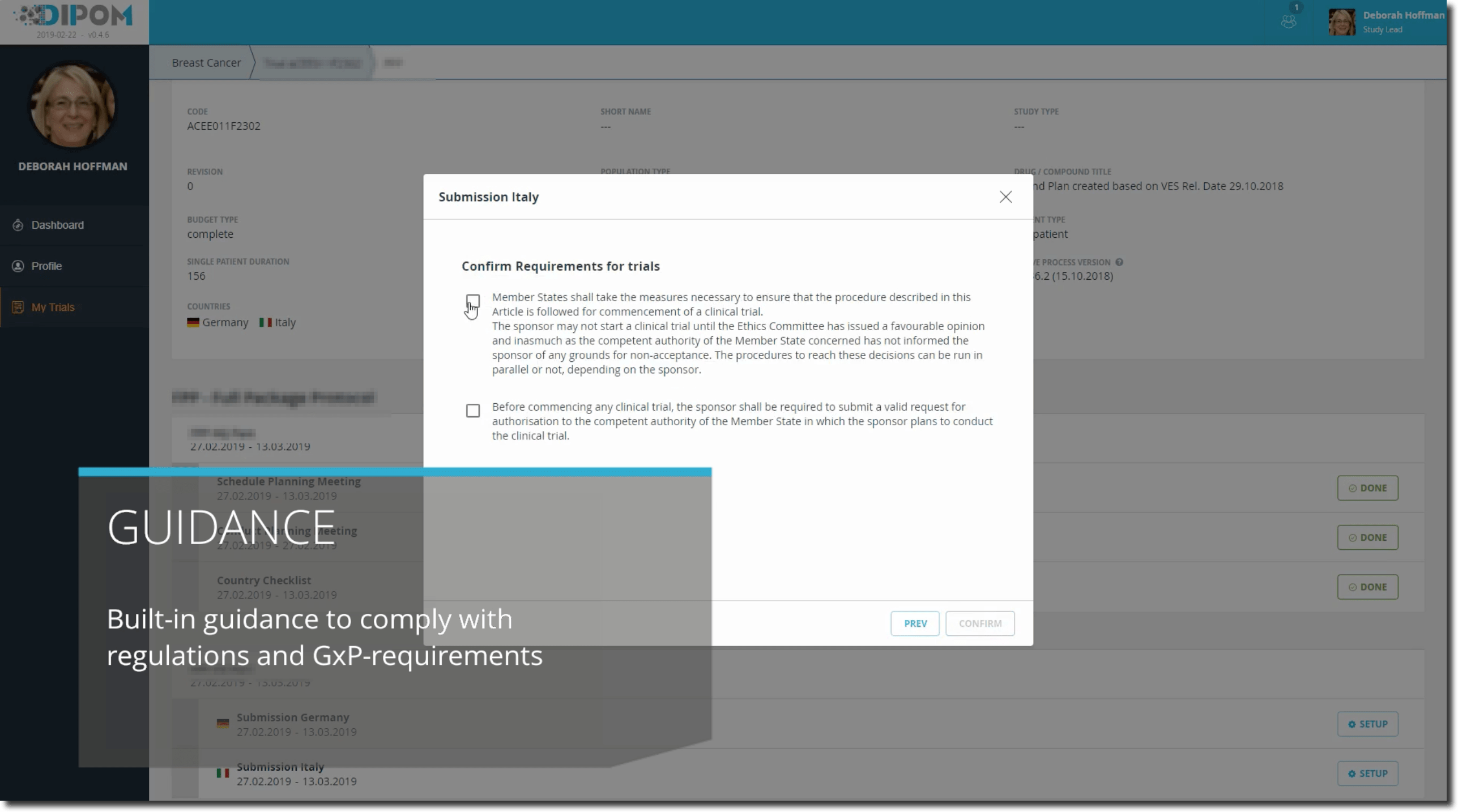
At the same time, in the past week, a small change in the overall trial submissions process has been endorsed. It was a minor change incorporated directly into all running processes. Deborah notices that the IT system is now asking for an additional information while she is conducting the business process. Other than that, she doesn’t need to care. She knows that in the upcoming audit, this information will show in the laid out process map and the relation to the respective regulatory requirement will show.
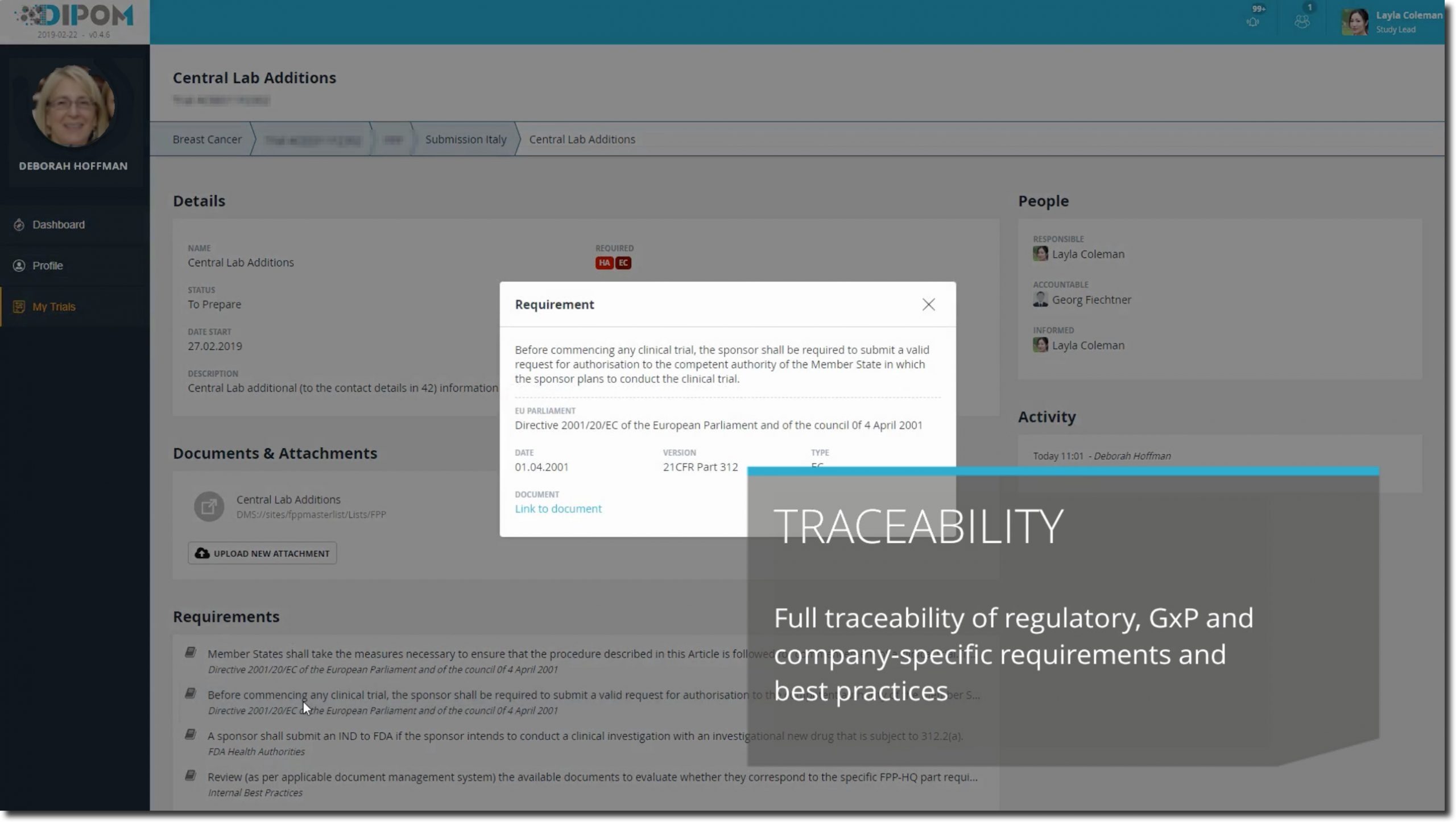
Added value
In addition to relieving employees from unnecessary trainings and ensuring full accountability of the company towards regulatory authorities such as EMA and FDA, further opportunities to increase operational effectiveness and efficiency were reaped:
- Playful implementation of each employee’s daily work routines through digital business processes
- The harmonization of the various role definitions within the organization layed the foundation for a professional competence management and rapid impact analysis in strategic decisions
- Better and targeted utilization planning of employees through integration of data on real-time availabilities
- Increased value contribution of the corporate IT systems through end2end integration of currently isolated systems.
Within the project, a concept for the integration of the – at the time isolated – five operational areas was developed. The concept served as the basis for creating so-called „wireframes“. The objectives of these was to simulate software solutions for the purpose of illustrating a concept but keeping IT programming costs low at the same time.
Conclusion
The concept presented in this article enables pharmaceutical companies to tap into numerous improvement and optimization potentials through targeted training and the interlinking of data points across various levels of the operating model. This means considerable time and cost advantages as well as the opportunity to further strengthen the corporations competitive advantage. At the same time, existing human resources can be used much better and more efficiently. Furthermore, pharmaceutical companies are significantly safer with regard to the implementation of legal regulations and reliable compliance. Moreover, the concept can be quickly and seamlessly integrated into existing IT system landscapes of pharmaceutical companies. Our project experience shows that chances for a successful transformation and acceptance of the concept and solution by employees are particularly good.
Please note, that all information presented in this case study is purely fictitious and has been significantly changed for demonstration purposes only, so that no conclusions can be drawn about our client and no confidential content is conveyed.
Are you facing similar challenges in your company? Do you have any questions about the case study described? If you are interested in an in-depth discussion, then please do not hesitate to contact us. We would be happy to present our consulting products and digital solutions to you in a personal discussion. We look forward to meeting you!
Related links
crosslync.smartlab: Eine smarte Plattform zu Digitalisierung des Laborbetriebs
Fallstudie: Training mit Virtual Reality & Avatar
Anwendungsbeispiele zur Zukunft der digitalen und virtuellen Arbeit mit Virtual Reality (VR)
Trends in Data-Driven Internet of Things (IoT)
Health 2.0: Digitale Gesundheitsversorgung
Fachkompetenz strukturelle Ausrichtung
Branchenkompetenz Gesundheitswesen
About us
Our positioning and mission statement: “Mastering the Digital Age!” We are an integrated management and technology consulting group and offer the conception and implementation of holistic digitization solutions in an integrated manner. In combination, you will receive strategic advice from us on the digitization of your company from real thought leaders with profound methodological knowledge, extensive transformation experience from successful IT system integrators with many years of industry experience and a high level of technological understanding, as well as deep expertise in generating value from data – the new gold of the 21st century – with the help of the latest artificial intelligence (AI) and cloud computing technologies. All this End2End from a single source for the entire transformation process! Find out more about us here.
Did we spark your interest?
Are you facing similar challenges in your company? Do you have any questions about the case study described? If you are interested in an in-depth discussion, then write us an email or give us a call. We would be happy to present our consulting products and digital solutions to you in a personal discussion. We are looking forward to meet you!

Marc R. Esser
Managing Partner
Strategy & Transformation Consulting
Email senden

Peter Wyser
Partner, Healthcare Practice
Strategy & Transformation Consulting
Email senden

Kommentare geschlossen